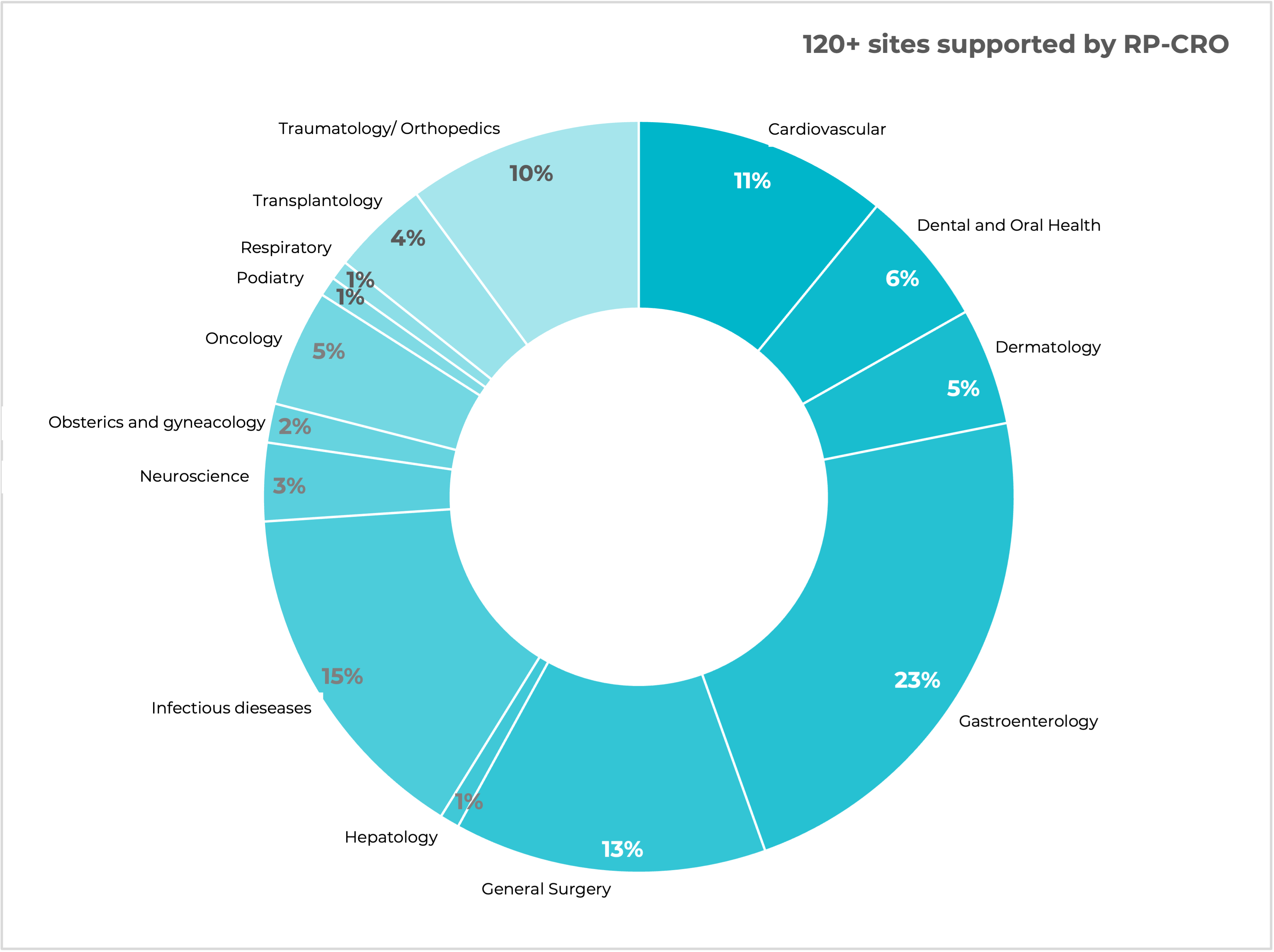
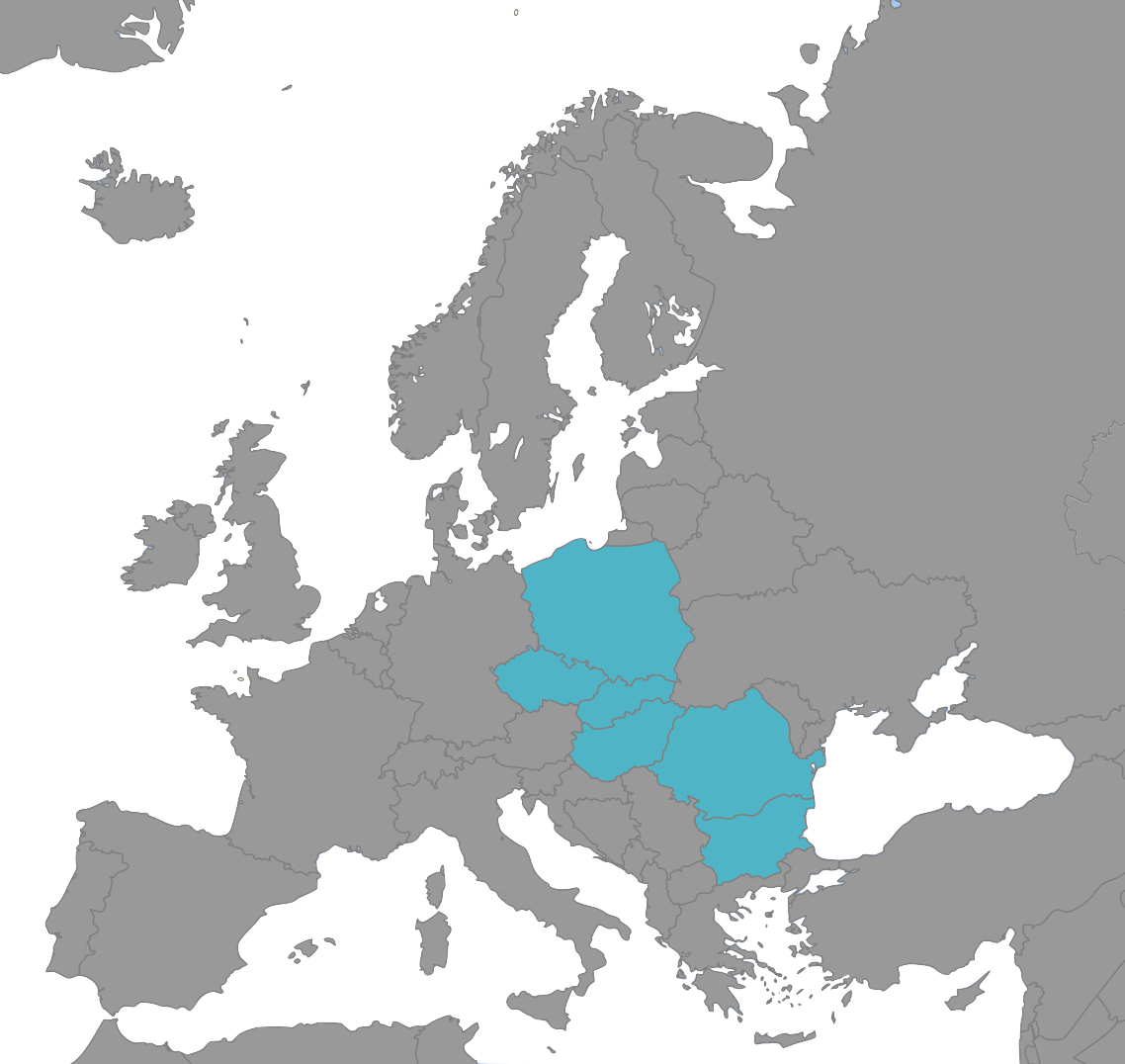
A leading GCP compliant CRO based in Hungary with operations in Poland, Czechia, Romania and Bulgaria, serving customers from across Europe and the globe.
Customers come to Research Professionals CRO (RP-CRO) from across the globe for our dedication to delivering the most efficient, flexible and highest quality GCP compliant CRO services. RP-CRO leverages its extensive network of highly experienced Principal Investigators and their large local subject pools to accelerate study enrolment for a wide range of study types. Our CRO team focusses on efficiently driving your study to completion and preparing the highest-quality submissions for the regulatory authorities.
Global Allianceof CROs
Research Professionals (RP) is the founder of ACROSS Global™, a strategic hub of CROs. We can cover 95+ countries, providing access to 7,500+ study sites, and plenty of potential clinical trial subjects.
HR search forclinical research
We ensure the fulfillment of our staff with a unique skill development tool. We help them in achieving goals and we support their personalized growth – step by step.
Next

HR search for clinical research
Global Alliance of CROs
Regional Coverage

HR search for
clinical research

Global Allianceof CROs

Regional Coverage

HR search forclinical research

Global Allianceof CROs

Regional Coverage
Putting the C in CRO
Clinical
We are experts in managing all phases of clinical studies for pharmaceuticals and devices. With clinical operations in Hungary, Poland, Czechia, Romania and Bulgaria as well as an ability to support Home Studies, RP-CRO can meet your clinical study requirements no matter what they are.
Capable
RP-CRO has the facilities and staff capable of delivering exceptional quality CRO services fast. We can assign our flexible internal resources to customer studies when and where they need them. This allows us to complete studies faster, to aggressive timelines without sacrificing quality.
Compliant
We strictly follow GCP regulations and use a CenterWatch based QMS resulting in high-quality submissions for our customers.
Cost-efficient
Headquartered in Eastern Europe as part of the EU, RP-CRO is able to deliver western market quality services for less. We leverage accelerated enrolment from large local subject pools, use leading PIs and flexible dedicated staff to reduce study timelines and program costs.
Compassionate
Our staff care about our study subjects and ultimately the patients that benefit from the drug or devices in our studies. For RP-CRO, taking care of the subjects in our studies means safe and compliant facilities, highly qualified medical staff and paying attention to the details. Our subjects matter.